Article from the first issue of Biomol Insight: Immune Checkpoints & Immunotherapy Research.
The aim of cancer immunotherapy is to mobilize the numerous components of the immune system in the fight against degenerated tumor cells. More specifically, immunotherapy maximizes the efficiency of antigen presentation and recognition mediated by dendritic cells and lymphocytes. Ideally, this approach achieves a more selective killing of cancer cells than would be possible with other therapeutic approaches, such as chemotherapy. Immune checkpoint therapy is a variant of cancer immunotherapy that targets the targeted activation of T cells (TCs). Activated T lymphocytes proliferate, produce various cytokines and can kill their target cells with high selectivity. Activation takes place via the binding of the T cell receptor (TCR) to the antigen-loaded AG / MHC complex (Major Histocompatibility Complex) of professional antigen presenting cells (APCs). The selectivity and strength of the activation is regulated by the influence of co-stimulating and inhibitory factors at the so-called immune checkpoints.
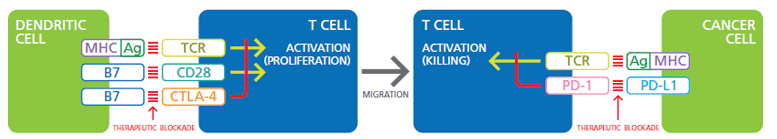
The immune checkpoint therapy uses the manipulation of the regulatory checkpoints to strengthen the T cell response against tumors. The interactions of the co-receptors that suppress TCR-mediated TC activation are currently best understood (see figure). An example of this is CTLA-4 (CTL-Associated Protein-4), an inhibitory co-receptor expressed on T cells, which, when stimulated simultaneously with the TCR, blocks T cell activation. By blocking the CTLA-4 signaling, CTLA-4-specific antibodies can therefore strengthen the T cell response against the tumor. This approach has already been used with limited but measurable success in the treatment of cancer patients, but the patients reacted only slowly and the effect was not always selective against tumor cells.1,2 A major obstacle here is that the CTLA-4 blockage occurs early T cell development must occur while the APCs stimulate T cells to proliferate. A better approach would be to attack if the T and tumor cells meet and the TCR initiates the cytotoxic program of the T lymphocyte.
PD-1 (Programmed Death-1) is a receptor expressed on lymphocytes which, after binding its ligands PD-L1 or PD-L2, inhibits TCR-mediated activation of the T cells and thus reduces their cytotoxic activity. The cells of some tumors take advantage of this by producing PD-L1, which, as expected, is associated with a significantly poorer prognosis for the patient. Various antibodies have therefore been developed over the past few years that block the PD-1-PD-L1 interaction, and the results of the clinical studies are promising. 3.4
Co-stimulation-of-TCR-and-CD28
Illustration. Co-stimulation of TCR and CD28 by Ag / MHC and B7 dentritic cells activates the proliferation and migration of T cells. The negative regulation of TCR by the B7: CTLA-4 signaling pathway can be blocked by therapeutic antibodies. The signal transmission from Ag / MHC via TCR works similarly in cancer cells, which leads to the cancer cells being killed. Blocking negative regulation by PD-L1: PD-1 is another goal of immunotherapy.
The examples of the checkpoint receptors CTLA-4 and PD-1 demonstrate the basic feasibility of this type of immunotherapy. Of course, the blockade of PD-1 only works if the tumor produces PD-1 ligands. For this reason, further inhibitory checkpoint receptors are constantly being identified (see table), and approaches that work with activating receptors are also being considered
Immunosuppression can of course also be effected by influencing important metabolic processes, for example indolamine deoxygenases (IDOs), arginase or via soluble factors such as TGF-β and adenosine.6 These signaling pathways can currently best be influenced by inhibitor molecules. Combination therapy with antibodies and inhibitors is expected to become a central element in the treatment of cancer.
Liganden-Rezeptor Paare mit Relevanz für die Immun-Checkpoint Therapie
| Antigen-presenting cell | T cell | ||
| Ligand / receptor | Also known as | Ligand / receptor | Also known as |
| CD40 | TNFRSF5 | CD40L | TNFSF5, CD154 |
| TL1A | TNFSF15 | DR3 | TNFRSF25 |
| GITRL | TNFSF18 | GITR | TNFRSF18, CD357 |
| 4-1BBL | TNFSF9, CD137L | 4-1BB | TNFRSF9, CD137 |
| OX40L | TNFSF4, CD252 | OX40 | TNFRSF4, CD134 |
| CD70 | TNFSF7, CD27L | CD27 | TNFRSF7 |
| HHLA2 | B7-H7 | TMIGD2 | TNFRSF14 |
| ICOSL | B7-H2, CD275 | ICOS | CD278 |
| CD80 | B7-1 | CD28 | |
| CD86 | B7-2 | ||
| MHC class I, II | KIR | ||
| TCR | |||
| LAG3 | CD223 | ||
| CD80 | B7-1 | CTLA-4 | CD152 |
| CD86 | B7-2 | ||
| CD80 | B7-1 | PD-L1 | B7-H1, CD274 |
| PD-L1 | B7-H1, CD274 | PD-1 | CD279 |
| PD-L2 | B7-DC, CD273 | ||
| PD-L1 | B7-H1, CD274 | CD80 | B7-1 |
| VISTA (2) | B7-H5, SISP1 | – | |
| BTNL2 (2) | – | ||
| B7-H3 | CD276 | – | |
| B7-H4 | VTCN1 | – | |
| CD48 | BCM1 | – | |
| Phosphatidylserine | TIM-3 (2) | HAVcr-2, CD366 | |
| Gal9 | LGALS9 | ||
| HVEM | TNFRSF14, CD270 | BTLA | CD272 |
| CD160 | |||
| LIGHT | TNFSF14, HVEML, CD258 |